Important changes are taking place in clinical research to make studies conducted in the EU more attractive. Of utmost importance is the launch of the new Clinical Trails Information System platform.
From the cycle of clinical trials - new regulations and important dates

Published April 6, 2023 13:41

Part 1 - the provisions of EU regulations and the new law of March 9 on clinical trials of medicinal products for human use.
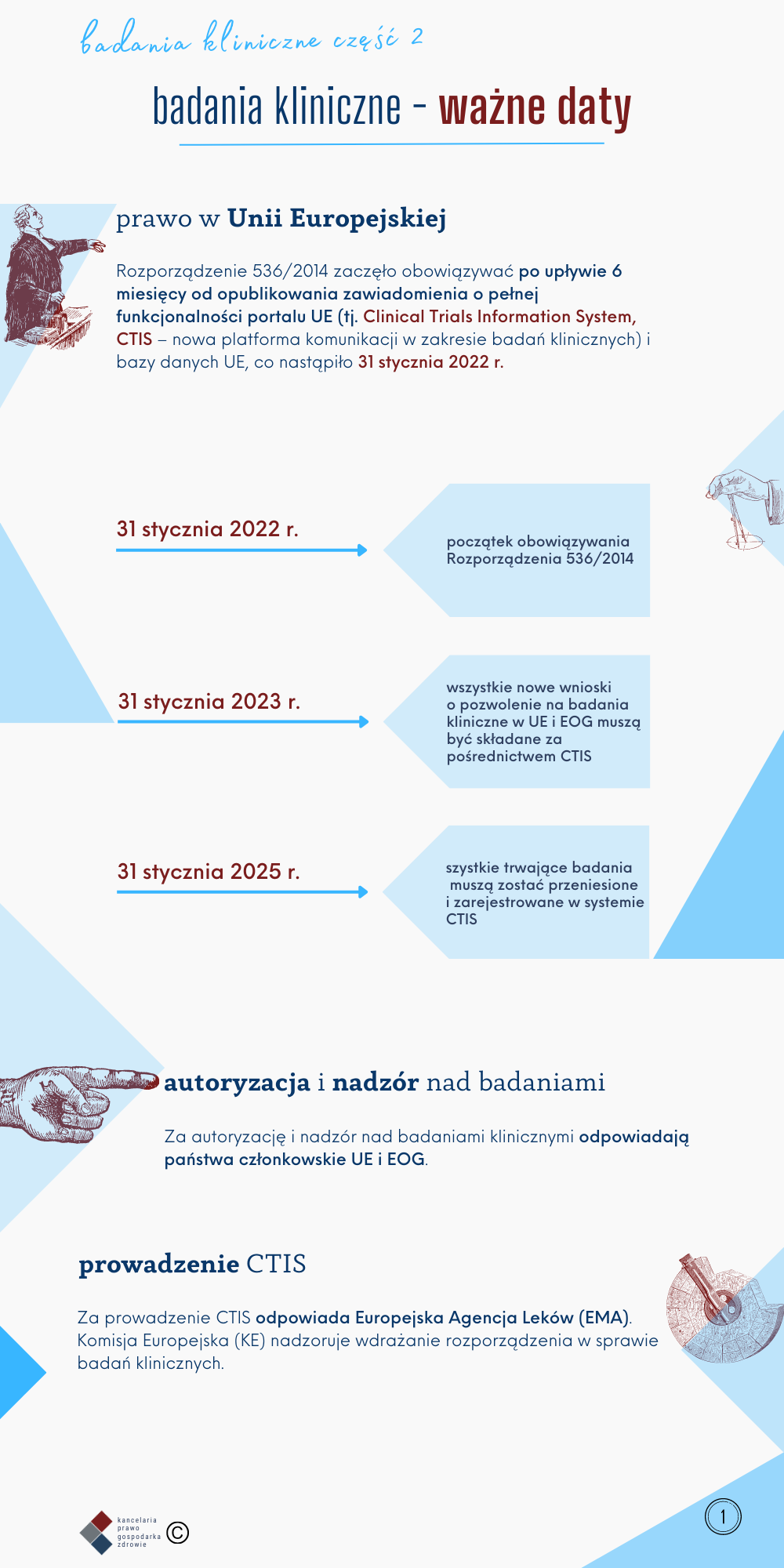
Part 2 - what are the most important dates related to the conduct of clinical trials?
Topics
badania kliczne














