Scientific advice from the president of URPL. To what extent and at what price?

Published May 30, 2023 08:00

The possibility for the President of the Office to provide scientific advice is a new institution, introduced into the Polish legal order by the Act of March 9, 2023 on clinical trials of medicinal products for human use (Journal of Laws, item 605) through amendments to the provisions of the Act of March 18, 2011 on the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products.
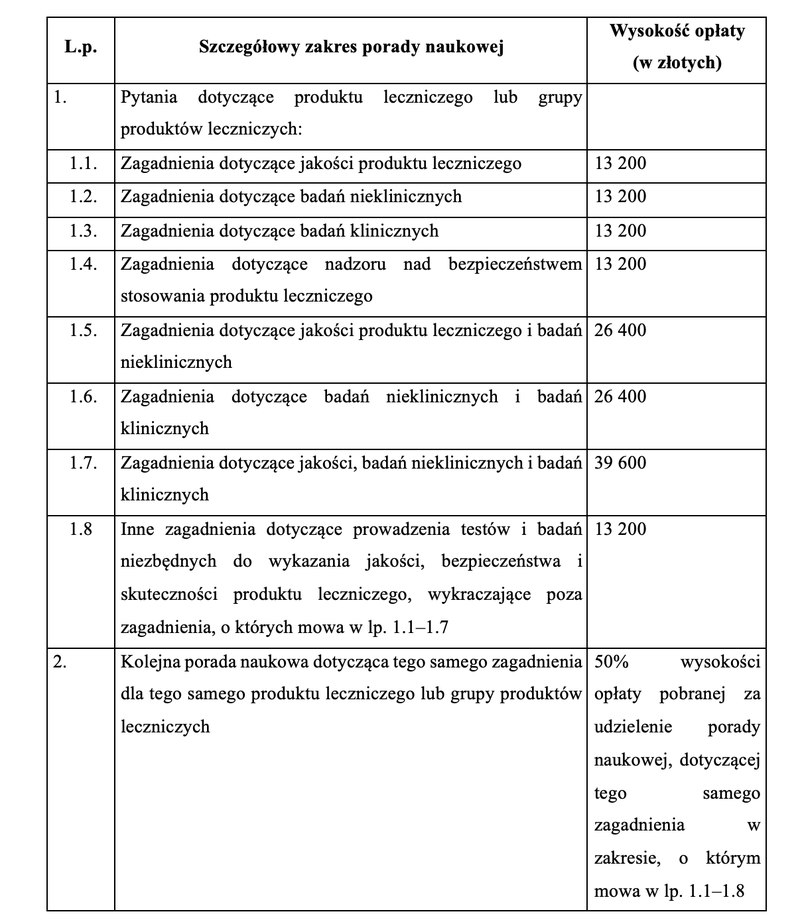
Annex 1 to the Regulation specifies the detailed scope of scientific advice provided by the President of the Office, along with the amount of fees specified for each scope of advice provided. In determining the amount of fees, the amount of work involved in providing advice and the level of costs incurred by the Authority were taken into account. A model application for scientific advice was also specified in order to streamline the procedure related to the provision of such advice.
The proposed solutions are modeled on the scientific advice provided by the European Medicines Agency (EMA), as set forth in Regulation (EC) No. 726/2004 of the European Parliament and of the Council of March 31, 2004 establishing EU procedures for the authorization and supervision of medicinal products for human use and establishing a European Medicines Agency.
Source: RCL