AOTMiT: list of highly innovative drug technologies

Published March 20, 2024 09:40

The prepared list will become the basis for further work on the reimbursement of drug technologies included in the list.
The creation of a list of highly innovative drug technologies (TLIs) is a systemic solution aimed at supporting efforts to improve health and quality of life.
The drugs evaluated were those for which the decision on marketing authorization in the central procedure in the European Union (EU) was issued from January 1, 2023 to December 31, 2023, for which marketing authorization decisions were published by 31.12.2023). According to a compilation by the European Medicines Agency (EMA), 60 medicinal products were authorized in the EU during the aforementioned period.
Drugs that received marketing authorization based on documentation of bioequivalence or biosimilarity were excluded from the process. The remaining 45 drug technologies are original drugs.
Of the 45 original medicines that qualified for further selection, products for which the marketing authorization decision covers use in oncology or rare diseases and for which no application for reimbursement and setting the official selling price had been submitted by the date of publication of the TLI list were evaluated.
A total of 23 medicinal products met the selection criteria for evaluation, most of which are drugs with an indication in oncological diseases (about 70%). Some of the drugs are products registered in oncology diseases with concomitant orphan drug status (about 38%).
In the evaluation process, one drug lost its orphan drug status, and was therefore excluded from the evaluation.
In addition, according to information received from the Ministry of Health (as of February 29, 2024), a reimbursement application had been submitted for four drugs, so they did not meet the evaluation criteria under TLI.
Of the products meeting all selection criteria in total (18), two drugs had more than one indication to be evaluated and were evaluated separately in each of the registered indications. As a result, 20 drug technologies were evaluated.
According to the analysis, some of the assessed technologies do not have an alternative technology funded in the assessed indications, which can be taken as the existence of an unmet medical need, although it should also be emphasized that the mere availability of a technology in a given indication is not tantamount to meeting the medical need in a given disease entity.
During the evaluation, special attention was paid to the quality of the available scientific evidence, which is related to the reliability of the results analyzed and affects the (in)certainty of the inference. And the health effects of the therapies were assessed with a view to whether the endpoints are primary or surrogate endpoints, as well as whether the difference in health effect size between the intervention and the comparator is significant.
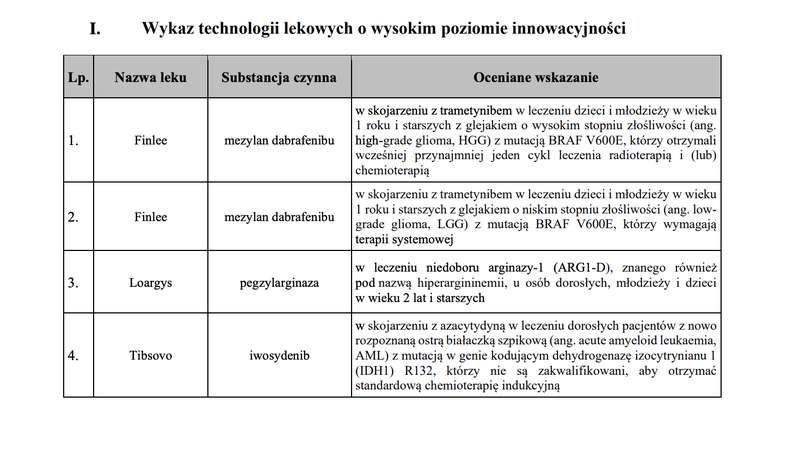
Based on the assessment of drug technologies, taking into account the statutory criteria, a TLI list was created, which includes 4 drug technologies.
The list is the first stage of
The creation of the TLI list is the first stage of the reimbursement process for highly innovative drug technologies. The next is the creation of the TLI list by the Minister of Health after prior consultation with the Transparency Council, National Consultants and the Patient Ombudsman. Placing a technology on the Minister of Health's TLI list allows the technology to move on to the next stage, which is the submission, in accordance with Article 24(1)(1b) of the Reimbursement Law, of applications for inclusion of the technology in reimbursement.
Source: AOTMiT:
Topics
Fundusz Medyczny















