Poland's historic advance in terms of access to innovative drugs

Published April 28, 2023 11:36

W.A.I.T. (Waiting to Access Innovative Therapies) is an annual study by the European Federation of Pharmaceutical Industries and Associations that analyzes waiting times for patients to access innovative therapies.
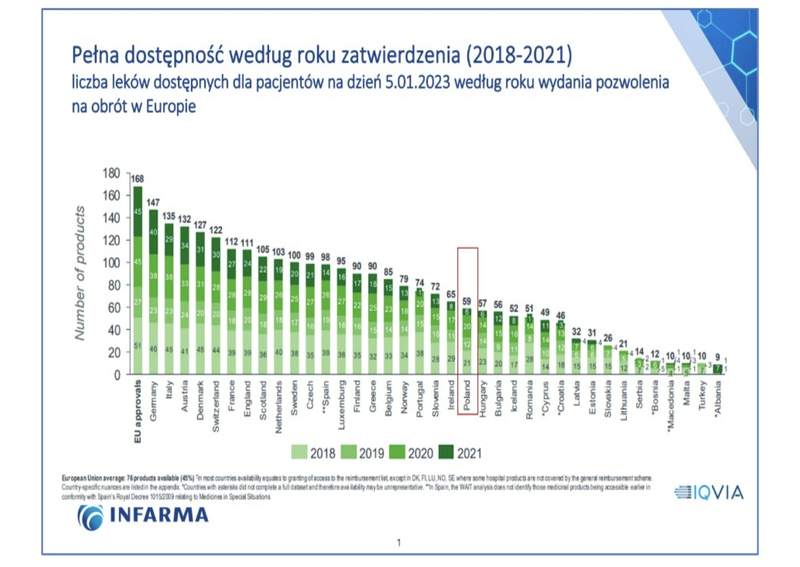
The latest list, just published, compared access to 168 innovative therapies in 37 European countries. Poland recorded the biggest promotion in the nearly 20-year history of W.A.I.T. - moving from 25th to 21st place in terms of drug availability. 35% of the innovative therapies analyzed are available in our country. This is a significant change from last year's results, according to which only 26% of therapies could be used for Polish patients. Germany remains the leader, with 147 available therapies. The other positions on the podium are occupied by Italy and Austria. In the overall ranking, Poland is ahead of, among others, two Visegrad Group countries - Hungary and Slovakia - and the Baltic countries - Lithuania, Latvia and Estonia. However, the rate of full accessibility shows areas for further improvement, with as many as 78% of the 59 therapies reimbursed in Poland for only a limited group of patients.
Polish patients are still waiting a long time for new therapies. Although there is a noticeable improvement in this aspect as well, but only by 17 days. Today, it takes 827 days from the moment a drug is registered to its entry into the reimbursement list or drug program in Poland. Against the background of 37 European countries, we rank 4th from the end, ahead of only Bosnia, Romania and Malta. By comparison, a patient in Germany waits only 128 days for the opportunity to use a new drug, in Denmark 191 and in Austria 301.
- The direction of change expected by the pharmaceutical industry, but also by the healthcare sector as a whole, is to continue successively closing the gaps in patients' access to therapies on a European basis, while keeping a close eye on how accessible the therapies introduced are in real terms to all patients in a given country. As Poland, we have seen the biggest advance in the EFPIA study's nearly 20-year history to date - both in terms of the number of reimbursed therapies and the time it takes from a drug's registration to its inclusion on the reimbursement list. Now it is important to maintain the momentum of these positive developments. Innovations in health care and increasing Polish patients' access to them are fostered by stable legislative conditions and predictability of the health care system. As we can see, the functioning legislative framework and market mechanisms, in addition to the openness of the public administration to talks with representatives of the pharmaceutical sector, allow a fuller use of the available tools, and give patients wider access to drug innovations," says Michal Byliniak, director general of the Association of Employers of Innovative Pharmaceutical Companies INFARMA.
The time at which innovative therapies are made available to patients remains a challenge in Europe. A patient in Malta can benefit from a therapy 1223 days, more than three years later than a patient in Germany. The differences are due to different health care systems and reimbursement processes. The pharmaceutical industry, sharing EFPIA's concerns, points out that the situation can be improved by solutions introduced at the European level. This is to be served, among other things, by the obligation of EFPIA member companies to submit reimbursement applications in all EU countries as soon as possible and no later than 2 years from the date of release of the drug on the market in the central procedure, provided that local systems allow it. The implementation of this commitment is to be facilitated by the launch of an online portal where the marketing authorization holder can provide timely information on the timing and processing of pricing and reimbursement applications in the EU-27.
The results of the W.A.I.T. study demonstrate inequalities in access to innovative treatment in different European countries - often with different systems for making new therapies available and at different stages of economic development. The analysis of access to innovative medicines in the Visegrad Group countries - that is, Poland, the Czech Republic, Slovakia and Hungary - is provided by the Access GAP platform https://gapv4.eu/pl/, which measured differences in access to innovative medicines and diagnostics in selected therapeutic areas in the V4 countries.
The report also found that one of the weakest points in access to innovative therapies is the percentage of available drugs among those recommended in the clinical guidelines between 2010 and 2021. In each of the diseases analyzed, limitations were diagnosed in access to the latest therapeutic options, but also to rapid diagnosis. This means that patients in the Visegrad countries are not being treated optimally in accordance with clinical guidelines. Challenges related to reimbursement procedures and timely access to diagnosis are areas where the Visegrad Group should act together.
Updated data for the Visegrad countries available on the Access GAP platform and a new tool for ongoing analysis of reimbursement announcements will be unveiled in May.
Source: INFARMA