There is a list of drug technologies with a high level of innovation

Published March 31, 2025 10:07

The prepared list will become the basis for further work on the reimbursement of drug technologies included in the list.
The creation of a list of highly innovative drug technologies (TLI) hereafter referred to as the "TLI list" is a systemic solution aimed at supporting efforts to improve health and quality of life.
Process of selecting drug technologies for evaluation
Drugs for which the decision on marketing authorization in the central procedure in the European Union (EU) was issued from January 1, 2024 to December 31, 2024, for which marketing authorization decisions were published by 31.12.2024, were evaluated.
According to a summary by the European Medicines Agency (EMA), 97 medicinal products were approved for marketing in the EU during the aforementioned period. In addition, three medicinal products registered in 2023, for whichdocumentation on the EMA website was available in 2024, qualified for evaluation).
Drugs that received marketing authorization based on documentation of bioequivalence or biosimilarity were excluded from the process. The remaining 56 drug technologies are original drugs.
Of the 56 original medicines that qualified for further selection, products for which the marketing authorization decision includes use in oncology or (products with orphan drug status) and for which no application for reimbursement and setting the official selling price had been submitted by the date of publication of the TLI list were evaluated.
A total of 17 medicinal products met the selection criteria for evaluation, of which almost half (48%) were drugs with an indication in rare diseases. Some of the drugs were products registered in oncological disease with concomitantorphan drug status (33%).
In the evaluation process for one of the drugs, the marketing authorization was withdrawn at the request of the responsible party, so it was excluded from the evaluation.
In addition, a reimbursement application has been submitted for ten drugs, which is an exclusion criterion for evaluation under TLI.
Of the products meeting all selection criteria in total (17), four drugs had more than one indication to be evaluated and were evaluated separately in each of the registered indications. Consequently, 21 drug technologies were evaluated.
Evaluation of drug technologies for the TLI list
Drug technologies with a high level of innovation are in the area of rare diseases and oncology, and the creation of the TLI list, focuses on promising therapies in these groups of diseases.
The criteria considered are the strength of the intervention, the quality of scientific data, the unmet health need, the size of the target population, and health priorities.
Based on the assessment of drug technologies, taking into account the aforementioned statutory criteria, a TLI list was created, which includes 6 drug technologies.
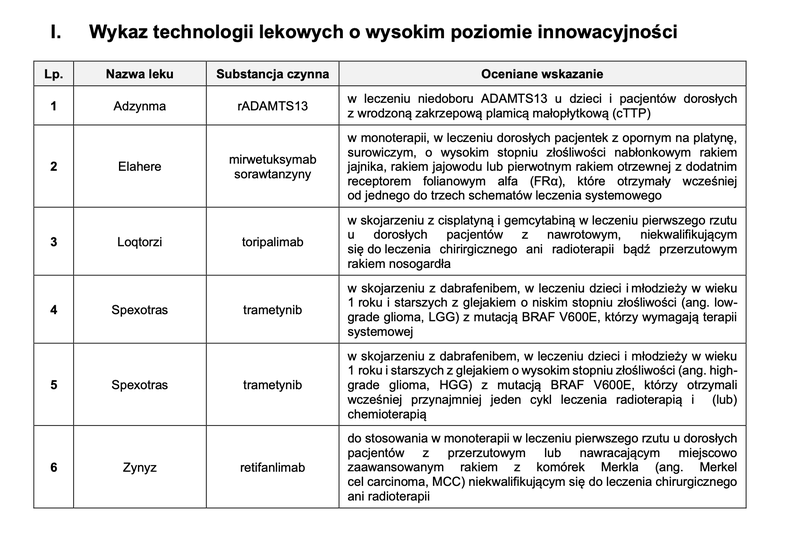
Source: AOTMiT