A lentiviral vector manufacturing facility opened at the Świętokrzyskie Oncology Center. This is the first step for Poland's CAR-T cell therapy

Published Oct. 13, 2023 14:10

The Świętokrzyskie Oncology Center is responsible in the CAR-NET project for preparing a lentiviral vector that will carry genetic information inside the patient's T lymphocytes. Based on this information, the lymphocytes will build a receptor on their surface so that they can recognize and destroy cancer cells.
Biotechnologists have harnessed the natural potential of lentiviruses (in this case HIV) to infect lymphocytes. The method is safe for the patient, because for the preparation of the lentiviral vector the active virus will not be used, but only its envelope (it is produced at the Plant in an incubator in the vector production stage), in which information in the form of DNA will be placed. The task of the vector is only to transfer the information inside the T lymphocytes, it is not capable of living and multiplying on its own in the patient's body. It is such a 'molecular porter'
- In practice, it will consist in the fact that in our Plant we will produce a lentiviral vector, i.e. a transporter in which we will encapsulate the genetic information. Then we will send this transporter to the centers, which will carry out the next step and use the vector for transduction, i.e. infection of T lymphocytes taken from the patient, under laboratory conditions, of course. On the basis of the genetic information provided, the patient's T lymphocytes will build a receptor on their surface, with the help of which they will recognize cancer cells and destroy them," explains Artur Kowalik, MD, UJK professor, head of the Department of Molecular Diagnostics, who directly supervises the Lentiviral Vector Manufacturing Plant.
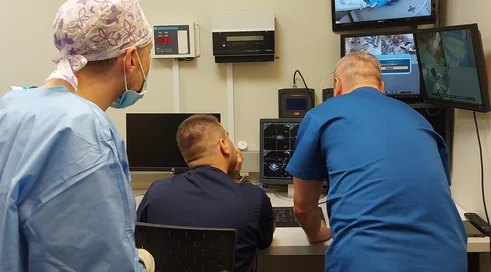
The operating plant at the ŚCO consists of a lentiviral vector manufacturing laboratory, a quality control laboratory and lagging rooms. Class B and C (the highest) cleanliness standards apply at this plant. Employees enter the plant and work only in special suits, masks and goggles. The reagents used have all purity certificates. All these are requirements for pharmaceutical production to ensure the highest quality and purity of the vectors produced.
Even garbage is sterilized before being thrown in the trash. Three biotechnologists (Dorota Kuc-Ciepluch, M.Sc., Anna Szymkiewicz, M.Sc., and Dawid Wisniewski, M.Sc.) are currently working in the laboratories of the Plant. The new R&D unit operates on the basis of pharmaceutical standards (GMP standard), and is subject to all regulations of pharmaceutical law, since its task is to produce an intermediate for the production of a living drug (CART cells).
The plant has already started trial production of vectors, and the next step is to test the product's potential, i.e. its ability to infect T lymphocytes. Readiness for full vector production is planned for November. Recruitment of patients for the clinical trial will begin in the second half of 2024.
The experience gained in the CAR-NET project will serve ŚCO specialists to develop the Molecular Research Center, which is being established at the ŚCO, where research will be conducted on new cellular therapies for cancer patients.
Adoptive therapy using genetically modified cells of the patient's immune system (T lymphocytes) to fight malignant tumors has been known worldwide for more than 10 years. It has been used successfully in hematology patients in whom standard treatment has failed. Currently, the cost of treating one patient with this method reaches $0.5 million (PLN 1.8 million). The Polish adoptive therapy, developed from scratch, can be up to 5 times cheaper.
In December 2020, the Medical Research Agency settled a competition for the development of a Polish adoptive therapy (CAR/CAR-T). The winning scientific and research team (consortium), which received a grant of PLN 100 million for the development of Polish CAR-T technology, as well as preclinical and clinical research, includes: Maria Sklodowska Curie National Cancer Institute, the University Clinical Center of Warsaw Medical University, the Institute of Hematology and Transfusiology, the Transfiguration Clinical Hospital of the Karol Marcinkowski Medical University in Poznan, the Dr. Antoni Jurasz University Hospital No. 1 in Bydgoszcz, the Karol Marcinkowski Medical University in Poznan, the Pomeranian Medical University in Szczecin, the Medical University of Lodz and the Swietokrzyskie Oncology Center. Within the framework of this project, the ŚCO received PLN 7 million from the Medical Research Agency for the establishment of the Lentiviral Vector Manufacturing Plant and for operation under the CAR-NET project.
Elaborated. Based on: Świętokrzyskie Oncology Center