Daratumumab in the DRd regimen reimbursed in the first line. It's a chance for up to five years of remission

Published Jan. 10, 2024 11:32

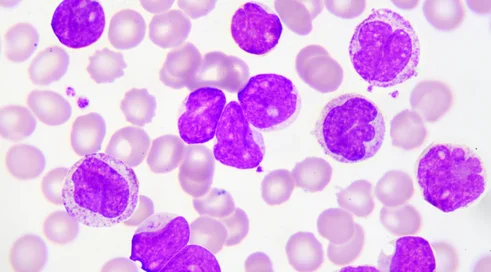
As of January 1, the plasmocytic myeloma drug program included daratumumab therapy in the first-line treatment regimen dRDd for patients ineligible for autologous hematopoietic stem cell transplantation. This is the regimen recommended by Polish and international guidelines. Prof. Krzysztof Giannopoulos of the Department of Experimental Hematooncology at the Medical University of Lublin is pleased with the decision.
- This was one of the reimbursement white spots. Daratumumab in the daraVTD regimen was available for transplant-eligible patients, and in the DVd and DRd regimens it was widely available for relapsed patients. The group of patients that is the most common, accounting for as much as 70 percent, those with plasmocytic myeloma who are not eligible for transplantation, could not benefit from daratumumab in the first-line treatment, the professor reports.
Meanwhile, this is crucial, for reasons of efficacy. Daratumumab has as a drug demonstrated efficacy in monotherapy, but in registration studies it has been shown to have the greatest efficacy in combination with immunomodulatory drugs or a proteasome inhibitor at the beginning of treatment.
- Recent years have shown exactly the same relationship in the DRd regimen. Patients treated in the relapse, or registration study, showed a median progression-free time of more than 40 months. In the registration study, in the first-line treatment, the median progression-free time was above 60 months. This reimbursement decision has therefore given patients an average of 20 more months of effective therapy. It allows us to optimally use daratumumab already in the first line of treatment and cover the entire population," explains Prof. Krzysztof Giannopuolos.
In practice, patients have gained the chance of an average five-year remission. This is especially significant if we note that the median time of onset, or diagnosis, of myeloma is 71 years.
- Of course, for individual patients, statistics are flawed. There will be those who will not require another therapy for up to 10 years, but also those who will need to be treated again after just a year or two. Undoubtedly, however, such good results in patients ineligible for transplantation have not yet been observed. The regimen that has been possible so far has had a median progression-free time of more than 40 months, and we were already very happy with this possibility," the professor admits.
Also of great importance from the point of view of patients is the method of administration of the therapy. Daratumumab is available there in the form of subcutaneous injections. Initially, these are administered weekly, and at a later stage of treatment the injections are taken once a month, in an outpatient-like setting. The remaining drugs are taken orally. This means that patients do not experience the inconvenience of the therapeutic process and can lead virtually normal lives during treatment.