Another version of the vaccine is authorized. Doctors call for clear guidelines

Published Sept. 13, 2022 09:53
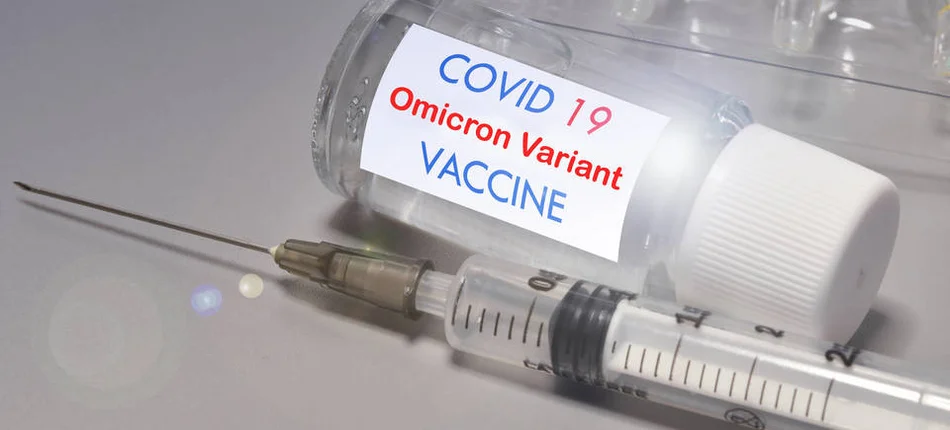
EMA's Committee for Human Medicines has recommended approval of an adapted bivalent vaccine against Omikron BA.4 and BA.5 sub-variants.
"This recommendation will further expand the arsenal of vaccines available to protect people from COVID-19 in the face of the ongoing pandemic and anticipated new waves of infection in the cold season," reads a press release on the agency's website.
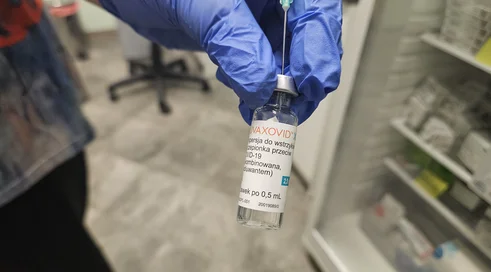
Comirnaty Original/Omicron BA.4-5 is a preparation intended for use in people 12 years of age and older who have received at least a basic course of vaccination against COVID-19. This vaccine is an adapted version of the Comirnata (Pfizer/BioNTech) COVID-19 mRNA vaccine.
Doctors, however, point to the problem that vaccination sites still have older versions of the preparations in stock, and the government has still not provided clear guidelines for further vaccination organization.
"The situation at the vaccination points is getting difficult, because there are vaccines from the 1st tranche (Pfizer, Moderna, Novavax), tomorrow the first delivery of vaccines from the 2nd tranche with the Omicron BA1 variant, and in a few weeks there will be vaccines from the 3rd tranche with the BA5 variant . We need clear guidelines! ", Dr. Paweł Grzesiowski, an infection prevention and therapy expert, wrote yesterday on Twitter.
Let us remind you that at the beginning of the week, the Minister of Health, Adam Niedzielski, announced universal vaccination with the fourth dose. There is still no specific date for extending the availability of the preparations. Now the second maintenance dose can be given to people over 60 years of age, chronically ill with severe immune disorders and medical staff.
Topics
EMA / Europejska Agencja Leków / COMIRNATY / szczepionka / szczepionka przeciw covid / omikron / omikron ba4. / omikron ba.5